Low-Temperature Chloride Corrosion in Boilers — Small Moisture, Big Damage
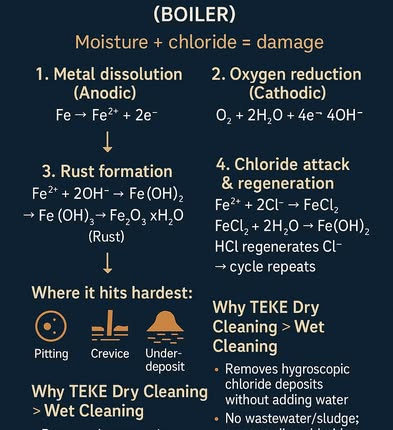
When hot gas meets a cool, slightly damp metal surface, moisture condenses and dissolves chloride salts/gases. That kick-starts a rapid corrosion cycle.
Role of Chloride Ion (Cl⁻) in Pitting (Quick Take)
Cl⁻ doesn’t oxidize iron directly; it breaks the protective Fe₂O₃ film, creating local defects → pitting
Cl⁻ penetrates crevices/under deposits, forming soluble iron chlorides → sustained metal dissolution
Key reactions:
Fe²⁺ + 2Cl⁻ → FeCl₂
FeCl₂ + 2H₂O → Fe(OH)₂ + 2HCl
→ HCl acidifies and regenerates Cl⁻, so the corrosion loop repeats
– Pitting on tubes/headers
– Crevice corrosion at gaskets, lap joints, clips
– Under-deposit corrosion beneath sticky ash/salts (economizer, air heater, SH/REH inlets)
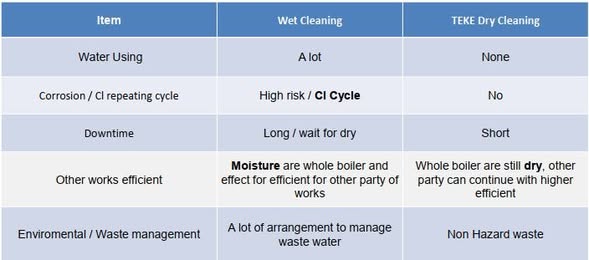
Why TEKE Cleaning (Dry) > Wet Cleaning  >
> 

Removes hygroscopic chloride deposits without adding water → less re-wetting/flash rust

No wastewater/sludge; no chloride-rich liquid seeping into crevices

Shorter downtime → faster return to service

Better long-term protection: clean, dry metal = fewer moisture/Cl⁻ cells
Message AGON PACIFIC to plan a TEKE Dry Cleaning for your boiler!